Axis formation-past research topics
Roles of maternal wnt8a transcripts in axis formation in zebrafish
In early zebrafish development, the program for dorsal axis formation begins soon after fertilization. Previous studies suggested that dorsal determinants (DDs) localize to the vegetal pole, and are transported to the dorsal blastomeres in a microtubule-dependent manner. The DDs activate the canonical Wnt pathway and induce dorsal-specific genes that are required for dorsal axis formation. Among wnt-family genes, only the wnt8a mRNA is reported to localize to the vegetal pole in oocytes and to induce the dorsal axis, suggesting that Wnt8a is a candidate DD. Here, to reveal the roles of maternal wnt8a, we generated wnt8a mutants by transcription activator-like effector nucleases (TALENs), and established zygotic, maternal, and maternal zygotic wnt8a mutants by germ-line replacement. Zebrafish wnt8a has two open reading frames (ORF1 and ORF2) that are tandemly located in the genome. Although the zygotic ORF1 or ORF2 wnt8a mutants showed little or no axis-formation defects, the ORF1/2 compound mutants showed antero-dorsalized phenotypes, indicating that ORF1 and ORF2 have redundant roles in ventrolateral and posterior tissue formation. Unexpectedly, the maternal wnt8a ORF1/2 mutants showed no axis-formation defects. The maternal-zygotic wnt8a ORF1/2 mutants showed more severe antero-dorsalized phenotypes than the zygotic mutants. These results indicated that maternal wnt8a is dispensable for the initial dorsal determination, but cooperates with zygotic wnt8a for ventrolateral and posterior tissue formation. Finally, we re-examined the maternal wnt genes and found that Wnt6a is an alternative candidate DD.
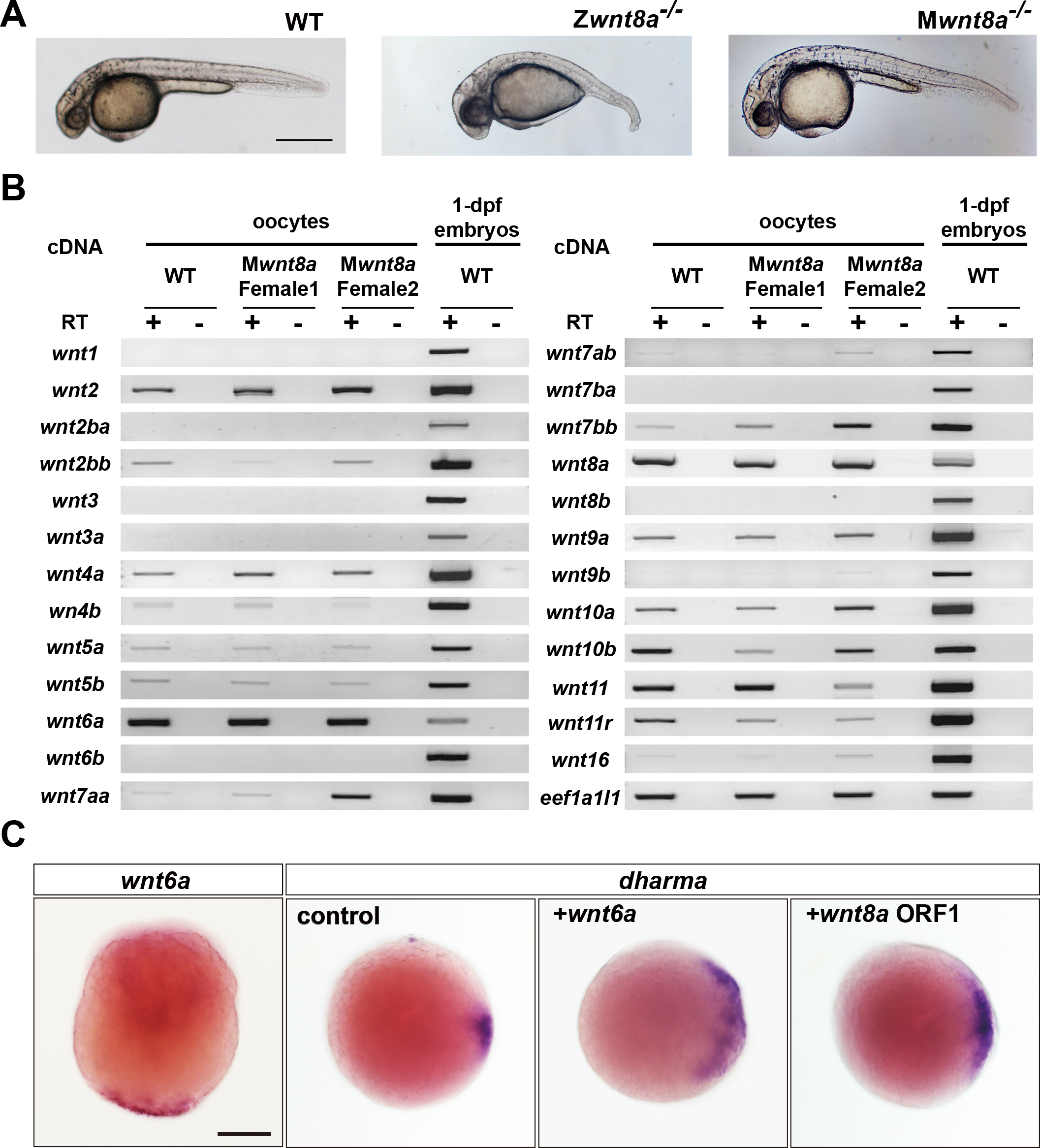
Phenotypes of wnt8a mutants and identification of a novel candidate for the dorsal determinants. (A) Wild-type (WT), zygotic (Z), and maternal zygotic (MZ) wnt8a mutant embryos at 1 day post fertilization (dpf). (B) Expression of wnt genes in wild-type and maternal wnt8a mutant oocytes and 1-dpf embryos. wnt6a is maternally deposited in oocytes. (C) Expression and function of wnt6a. wnt6a mRNA was detected at the vegetal pole of the yolk. Overexpression of wnt6a induced ectopic expression of dharma, as that of wnt8a did.
Dynein axonemal intermediate chain 2 is required for formation of the left-right body axis and kidney in Medaka
Ciliary defects lead to various diseases, such as primary ciliary dyskinesia (PCD) and polycystic kidney disease (PKD). We isolated a medaka mutant mii, which exhibits defects in the left-right (LR) polarity of organs, and found that mii encodes dynein axonemal intermediate chain 2a (dnai2a). Ortholog mutations were recently reported to cause PCD in humans. mii mutant embryos exhibited loss of nodal flow in Kupffer's Vesicle (KV), which is equivalent to the mammalian node, and abnormal expression of the left-specific gene. KV cilia in the mii mutant were defective in their outer dynein arms (ODAs), indicating that Dnai2a is required for ODA formation in KV cilia. While the mii mutant retained motility of the renal cilia and failed to show PKD, the loss of dnai2a and another dnai2 ortholog dnai2b led to PKD. These findings demonstrate that Dnai2 proteins control LR polarity and kidney formation through regulation of ciliary motility.
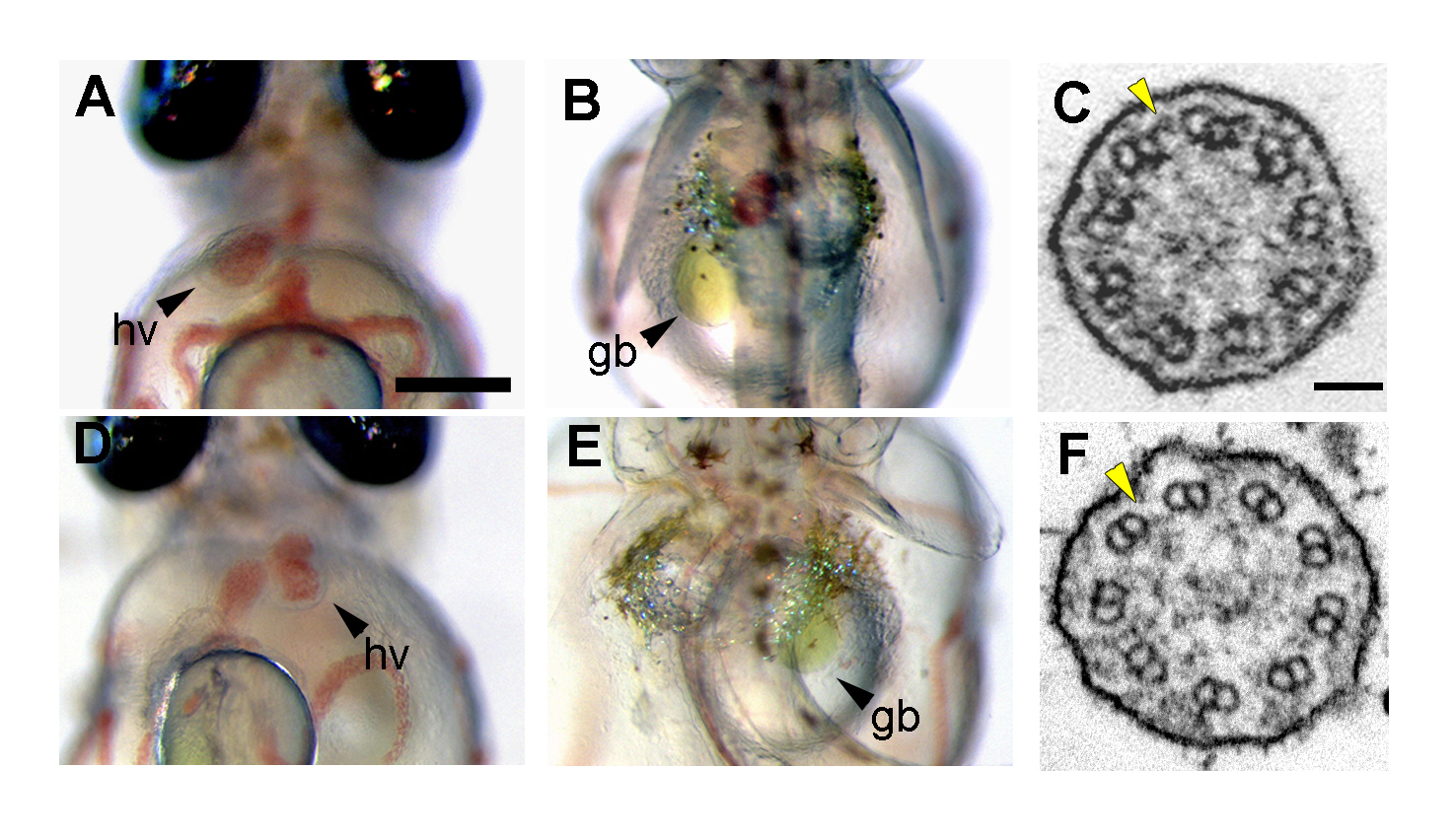
(A, B, D, E) Organ laterality at 7 days post fertilization (dpf). (A, B) In wild-type embryo, the heart ventricle (hv) and gallbladder (gb) were observed on the left when viewed ventrally and dorsally, respectively. (D, E) Some of the mii mutant embryos exhibited situs inversus. (C, F) Transmission electron micrographs of Kupffer’s vesicle (KV) cilia. (C) Cross-section of cilia in wild-type Kupffer’s vesicle showed a 9+0 axonemes with outer dynein arms (ODAs, C), indicated by yellow arrowheads. (D) ODAs were completely absent in KV cilia of the mii mutant embryos.