Functions of neural circuits-classical fear conditioning
Granule cells control recovery from classical conditioned fear responses in the zebrafish cerebellum [Pubmed]
Although previous studies show that the cerebellum is involved in classical fear conditioning, it is not clear which components in the cerebellum control it or how. We addressed this issue using a delayed fear-conditioning paradigm with late-stage zebrafish larvae, with the light extinguishment as the conditioned stimulus (CS) and an electric shock as the unconditioned stimulus (US). The US induced bradycardia in the restrained larvae. After paired-associate conditioning with the CS and US, a substantial population of the larvae displayed CS-evoked bradycardia responses. To investigate the roles of the zebrafish cerebellum in classical fear conditioning, we expressed botulinum toxin or the Ca2+ indicator GCaMP7a in cerebellar neurons. The botulinum-toxin-dependent inhibition of granule-cell transmissions in the corpus cerebelli (CCe, the medial lobe) did not suppress the CS-evoked bradycardia response, but rather prolonged the response. We identified cerebellar neurons with elevated CS-evoked activity after the conditioning. The CS-evoked activity of these neurons was progressively upregulated during the conditioning and was downregulated with repetition of the unpaired CS. Some of these neurons were activated immediately upon the CS presentation, whereas others were activated after a delay. Our findings indicate that granule cells control the recovery from conditioned fear responses in zebrafish.
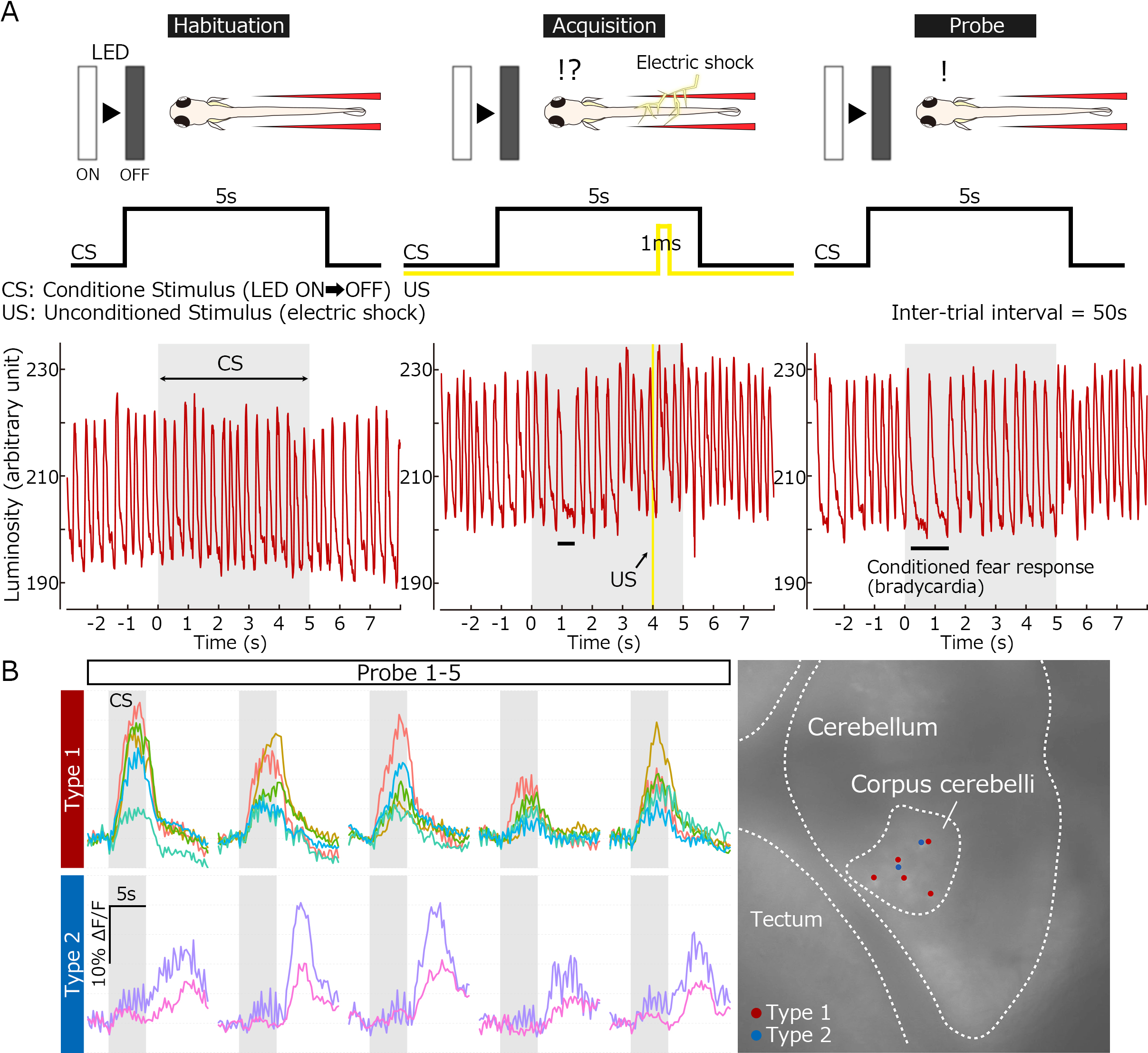
Classical fear conditioning in zebrafish. (A) Experimental paradigm for delayed classical fear conditioning. The extinguishment of a white LED light was used as the condition stimulus (CS), and an electric shock was used as the unconditioned stimulus (US). Before the conditioning, bradycardia responses were elicited only by the US. After the paired-associated learning, the conditioned bradycardia occurred shortly after the CS onset. (B) Conditioning-associated neurons. Ca2+ imaging with transgenic fish expressing GCaMP7a identified two types of cerebellar neurons whose activity was elicited by the CS upon the conditioning (early and late responders). They are located in the corpus cerebelli and likely granule cells.
Medaka and zebrafish contactin1 mutants as a model for understanding neural circuits for motor coordination [Pubmed]
A spontaneous medaka ro mutant shows abnormal wobbling and rolling swimming behaviors. By positional cloning, we mapped the ro locus to a region containing the gene encoding Contactin1b (Cntn1b), which is an immunoglobulin (Ig)-superfamily domain-containing membrane-anchored protein. The ro mutant had a deletion in the cntn1b gene that introduced a premature stop codon. Furthermore, cntn1b mutants generated by the CRISPR/Cas9 system and trans-heterozygotes of the CRISPR mutant allele and ro had abnormal swimming behavior, indicating that the cntn1b gene was responsible for the ro-mutant phenotype. We also established zebrafish cntn1a and cntn1b mutants by transcription activator-like effector nucleases (TALENs). Zebrafish cntn1b but not cntn1a mutants showed abnormal swimming behaviors similar to those in the ro mutant, suggesting that Cntn1b plays a conserved role in the formation or function of the neural circuits that control swimming in teleosts. Although Cntn1-deficient mice have abnormal cerebellar neural circuitry, there was no apparent histological abnormality in the cerebellum of medaka or zebrafish cntn1b mutants. The medaka cntn1b mutants had defective optokinetic response (OKR) adaptation and abnormal rheotaxis (body positioning relative to water flow). Medaka and zebrafish cntn1b mutants are effective models for studying the neural circuits involved in motor learning and motor coordination.
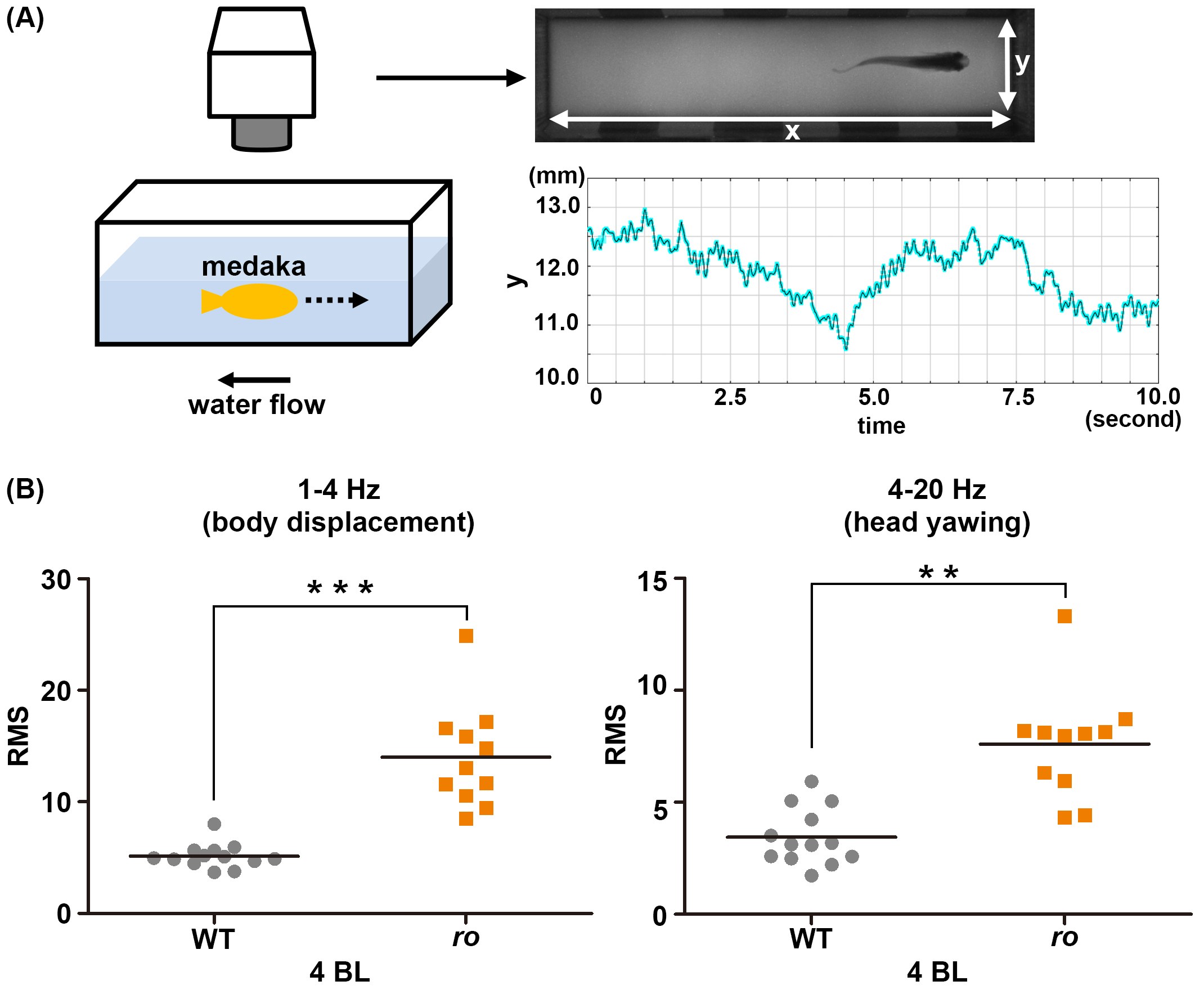
Cntn1b is required for controlling head position in rheotaxis. (A) Experimental setup for analyzing swimming behavior. An adult medaka fish was placed into a swim-mill chamber and the water flow was set to a velocity of 1, 2, 3, or 4 body lengths (BL)/s. The movements of the fish were recorded with a video camera. The head movements were tracked, and the y-axis movement (the direction perpendicular to the water flow) was plotted. The graph shows data from a representative wild-type medaka fish. (B) Medaka cntn1b (ro) mutants cannot keep a stereotaxic position while swimming into the water flow. The y-axis head movements were separated into low-frequency (1–4 Hz, stereotaxic change) and high-frequency (4–20 Hz, yawing) movements using Lab Chart software, and the root mean square (RMS) was determined for each component. The graphs here show the RMS for WT (gray circles, n=13) and ro-mutant (orange squares, n=11) medaka in a water-flow velocity of 4 BL/s. Head positioning during swimming was significantly affected in the ro-mutant compared to WT fish.