Neural development-formation of cerebellum and cerebellar neural circutis
Gene expression profiling of granule cells and Purkinje cells in the zebrafish cerebellum [Pubmed]
The structure of the neural circuitry of the cerebellum, which functions in some types of motor learning and coordination, is generally conserved among vertebrates. However, some cerebellar features are species specific. It is not clear which genes are involved in forming these conserved and species-specific structures and functions. This study uses zebrafish transgenic larvae expressing fluorescent proteins in granule cells, Purkinje cells, or other cerebellar neurons and glial cells to isolate each type of cerebellar cells by fluorescence-activated cell sorting and to profile their gene expressions by RNA sequencing and in situ hybridization. We identify genes that are upregulated in granule cells or Purkinje cells, including many genes that are also expressed in mammalian cerebella. Comparison of the transcriptomes in granule cells and Purkinje cells in zebrafish larvae reveals that more developmental genes are expressed in granule cells, whereas more neuronal-function genes are expressed in Purkinje cells. We show that some genes that are upregulated in granule cells or Purkinje cells are also expressed in the cerebellum-like structures. Our data provide a platform for understanding the development and function of the cerebellar neural circuits in zebrafish and the evolution of cerebellar circuits in vertebrates.
Transcriptome analysis of cerebellar neurons. Zebrafish transgenic lines expressing fluorescent proteins (A for granule cells, B for Purkinje cells) were used to isolated each type of cerebellar cells by fluorescence-activated cell sorting (FACS) and to profile their gene expressions by RNA sequencing and in situ hybridization. Example of genes upregulated in granule cells (C-F) or Purkinje cells (G-J).
Type IV Collagen Controls the Axogenesis of Cerebellar Granule Cells by Regulating Basement Membrane Integrity in Zebrafish [Pubmed]
Granule cells (GCs) are the major glutamatergic neurons in the cerebellum, and GC axon formation is an initial step in establishing functional cerebellar circuits. In the zebrafish cerebellum, GCs can be classified into rostromedial and caudolateral groups, according to the locations of their somata in the corresponding cerebellar lobes. The axons of the GCs in the caudolateral lobes terminate on crest cells in the dorsal hindbrain, as well as forming en passant synapses with Purkinje cells in the cerebellum. In the zebrafish mutant shiomaneki (sio), the caudolateral GCs extend aberrant axons. Positional cloning revealed that the sio gene locus encodes Col4a6, a subunit of type IV collagen, which, in a complex with Col4a5, is a basement membrane (BM) component. Both col4a5 and col4a6 mutants displayed similar abnormalities in the axogenesis of GCs and retinal ganglion cells (RGCs). Although type IV collagen is reported to control axon targeting by regulating the concentration gradient of an axonal guidance molecule Slit, Slit overexpression did not affect the GC axons. The structure of the BM surrounding the tectum and dorsal hindbrain was disorganized in the col4a5 and col4a6 mutants. Moreover, the abnormal axogenesis of the caudolateral GCs and the RGCs was coupled with aberrant BM structures in the type IV collagen mutants. The regrowth of GC axons after experimental ablation revealed that the original and newly formed axons displayed similar branching and extension abnormalities in the col4a6 mutants. These results collectively suggest that type IV collagen controls GC axon formation by regulating the integrity of the BM, which provides axons with the correct path to their targets.
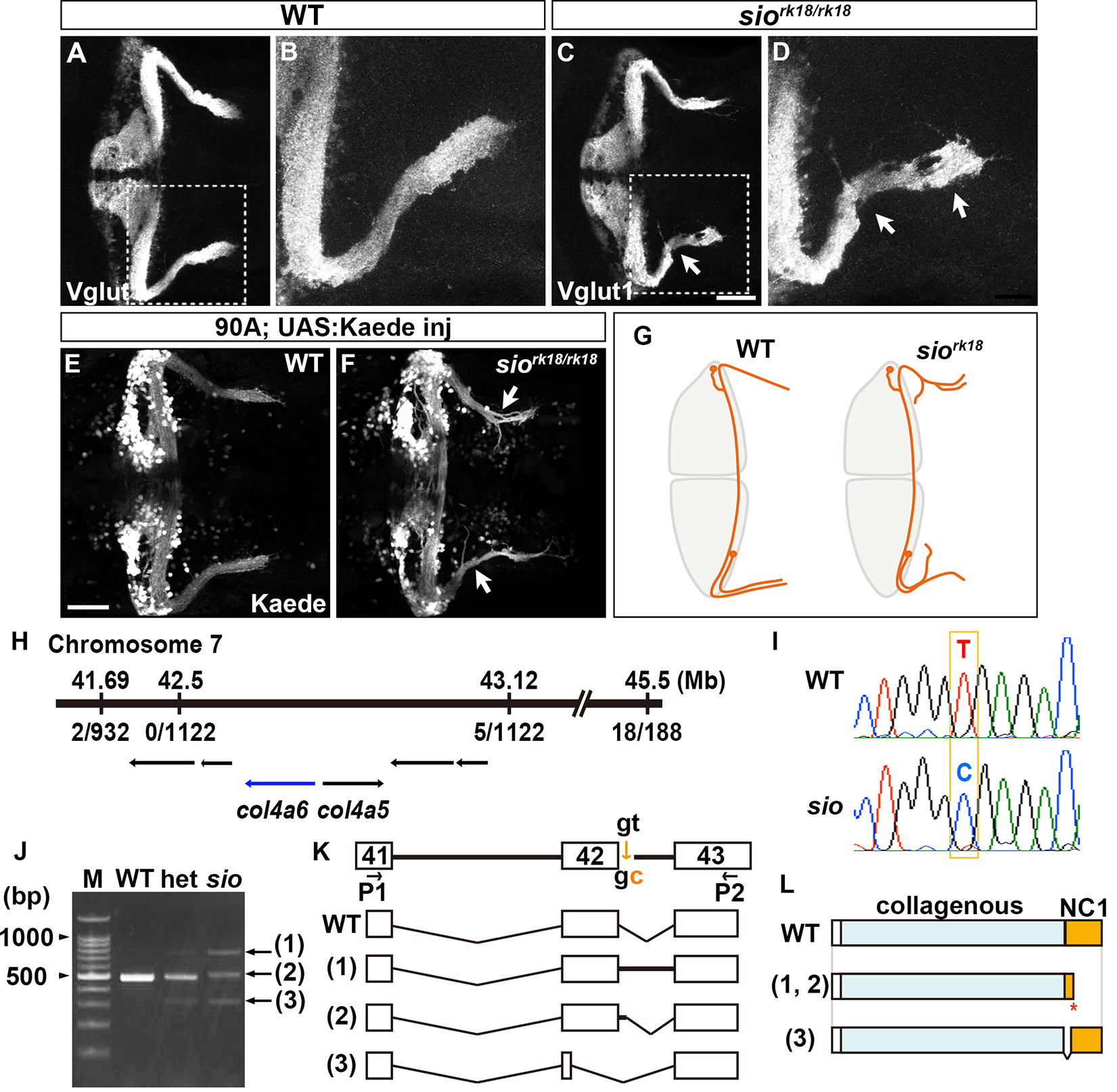
Type IV collagen gene col4a6 is required for axogenesis of the GCs in caudolateral lobes. (A-D) Staining with an anti-Vglut1 antibody, which marks the presynaptic termini of GC axons, revealed that the GCs in the caudolateral lobes of 5-dpf homozygous shiomaneki (siork18/rk18) mutants had abnormal axons, which formed abnormally branched bundles. (E, F) Labeling of the caudolateral GCs in the GC-specific Gal4 line hspGFFDMC90A also showed aberrant axogenesis (marked by arrowheads in F). (G) Schematic representation of sio phenotypes. (H-L) The sio locus encodes Col4a6. The mutant contained a T-to-C point mutation in a splicing donor site of the col4a6 gene, which generate the C-terminal truncated protein.
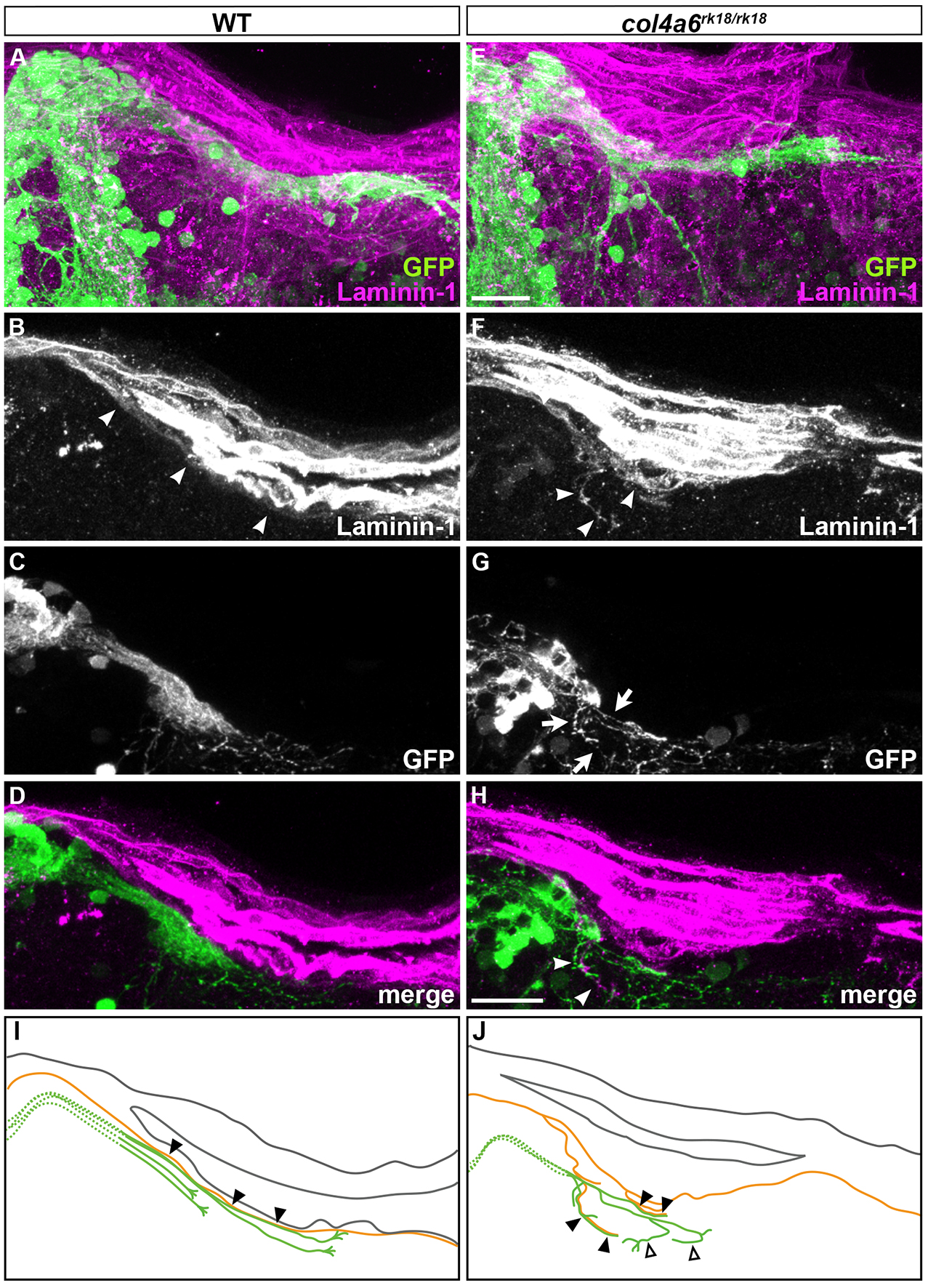
Abnormal BM structure is coupled with the abnormal axogenesis of GCs in col4a6 mutants. Wild-type (A-D) and col4a6 mutant (E-H) transgenic larvae that express GFP in the GCs were stained at 5 dpf with anti-GFP (green) and anti-laminin–1 (magenta) antibodies. Dorsal projection views. (I, J) Schematic representation of BM (brown) and GC axons (green) in wild-type and the col4a6 mutant hindbrain. The laminin–1+BM structure was split into two layers in the col4a6 mutant (indicated by arrows in G). In the same region, the corresponding GC axons were bifurcated, some of the axons ran along the split BM layers (indicated by arrowheads in F and H, by closed triangles in J) and some of them did not run along the BM layer (indicated by open triangles in J).
Establishment of Gal4 transgenic zebrafish lines for analysis of development of cerebellar neural circuitry [Pubmed]
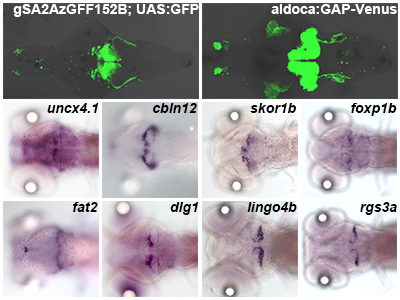
The cerebellum is involved in some forms of motor coordination and motor learning. Here we isolated transgenic (Tg) zebrafish lines that express a modified version of Gal4-VP16 (GFF) in the cerebellar neural circuits: granule, Purkinje, or eurydendroid cells, Bergmann glia, or the neurons in the inferior olive nuclei (IO) which send climbing fibers to Purkinje cells, with the transposon Tol2 system. By combining GFF lines with Tg lines carrying a reporter gene located downstream of Gal4 binding sequences (upstream activating sequence: UAS), we investigated the anatomy and developmental processes of the cerebellar neural circuitry. Combining an IO-specific Gal4 line with a UAS reporter line expressing the photoconvertible fluorescent protein Kaede demonstrated the contralateral projections of climbing fibers. Combining a granule cell-specific Gal4 line with a UAS reporter line expressing wheat germ agglutinin (WGA) confirmed direct and/or indirect connections of granule cells with Purkinje cells, eurydendroid cells, and IO neurons in zebrafish. Time-lapse analysis of a granule cell-specific Gal4 line revealed initial random movements and ventral migration of granule cell nuclei. Transgenesis of a reporter gene with another transposon Tol1 system visualized neuronal structure at a single cell resolution. Our findings indicate the usefulness of these zebrafish Gal4 Tg lines for studying the development and function of cerebellar neural circuits.
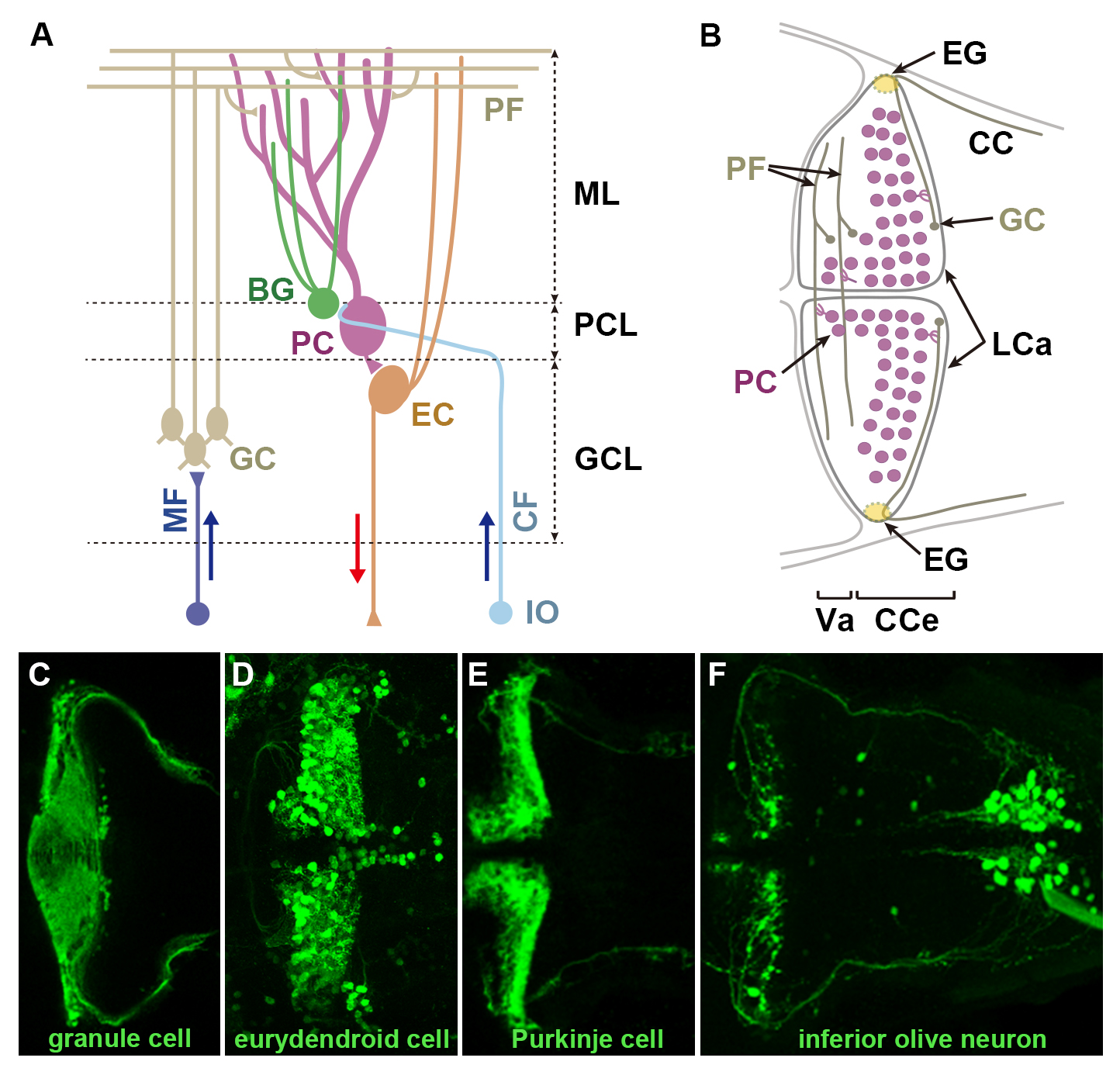
(A) Schematic representation of cerebellar neural circuitry. (B) Schematic drawing of the cerebellum at 5 days post fertilization (dpf). (C-F) Example of transgenic line isolated in this study. Transgenic lines expressing Gal4 in granule cells (C), eurydendroid cells (D), or neurons in the inferior olive nuclei (F), were crossed with UAS:GFP reporter fish. Purkinje cell-specific GAP-Venus line (E). 5 dpf. Dorsal views. BG: Bergmann glia, CC: crest cerebellaris, CCe: corpus cerebellaris, CF: climbing fibers, EC: eurydendroid cells, GC: granule cell, GCL: granule cell layer, IO: inferior olive nuclei, LCa: lobus caudalis cerebelli, ML: molecular layer, PC: Purkinje cell, PCL: Purkinje cell layer, PF: parallel fiber, Va: valvular cerebelli.
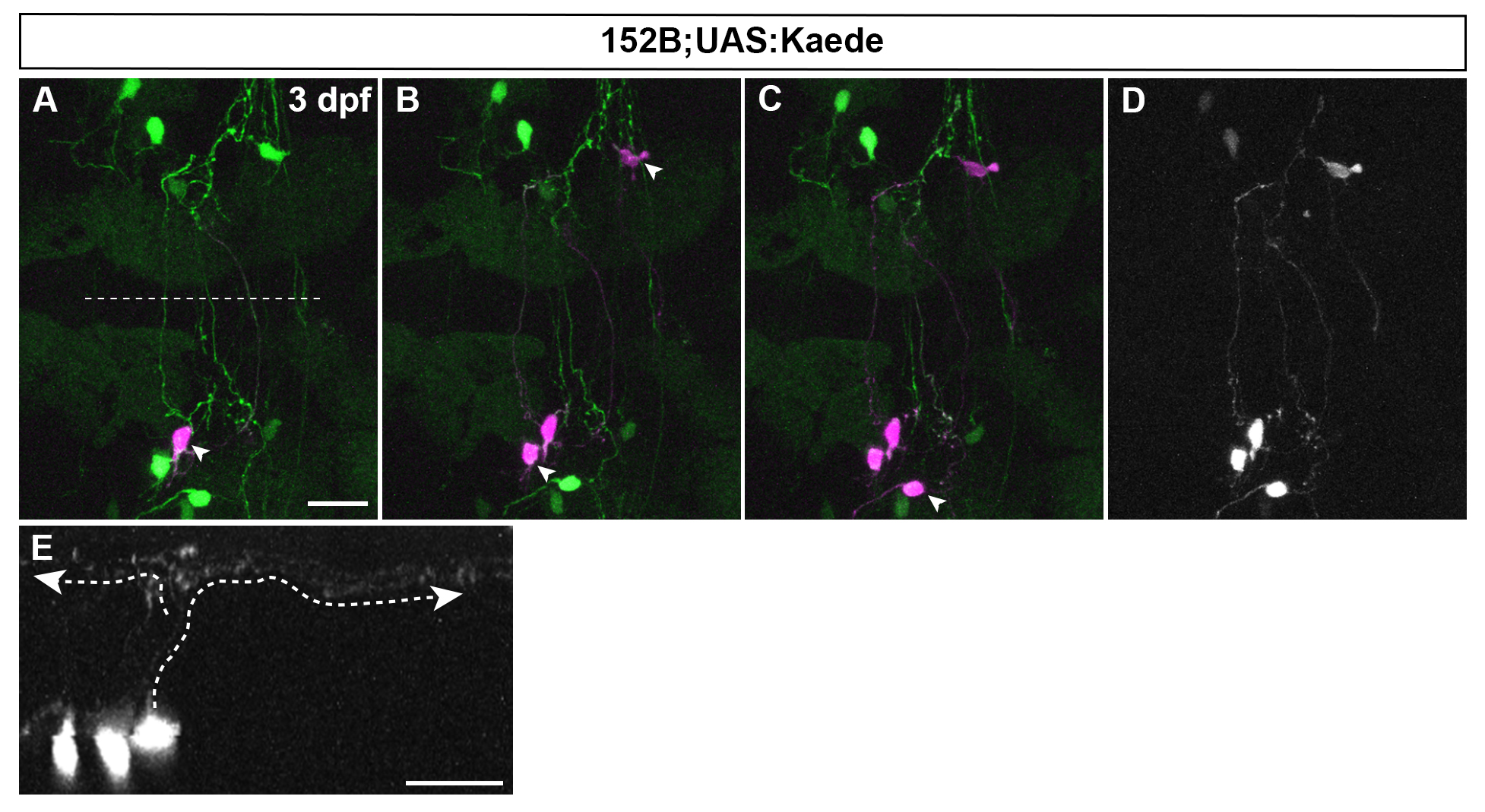
Combination of mosaic UAS:Kaede and laser irradiation shows the structure of granule cells at a single cell resolution. gSA2AzGFF152B line was crossed with mosaic (partially silenced) UAS:Kaede line. Single somata of granule cells were marked by laser-irradiation one by one at 3 dpf (one in A, three in B, and four in C, marked by arrowheads). Expression of original (green) and photoconverted Kaede (magenta) is shown in A-C. (D, E) Dorsal view and cross section image for photoconverted Kaede in C. Note granule cells in the corpus cerebelli shows typical T-shaped parallel fibers (marked by dotted lines with arrowheads in E).
Atypical protein kinase C regulates primary dendrite specification of cerebellar Purkinje cells by localizing Golgi apparatus [Pubmed]
Neurons have highly polarized structures that determine what parts of the soma elaborate the axon and dendrites. However, little is known about the mechanisms that establish neuronal polarity in vivo. Cerebellar Purkinje cells extend a single primary dendrite from the soma that ramifies into a highly branched dendritic arbor. We used the zebrafish cerebellum to investigate the mechanisms by which Purkinje cells acquire these characteristics. To examine dendritic morphogenesis in individual Purkinje cells, we marked the cell membrane using a Purkinje cell-specific promoter to drive membrane-targeted fluorescent proteins. We found that zebrafish Purkinje cells initially extend multiple neurites from the soma and subsequently retract all but one, which becomes the primary dendrite. In addition, the Golgi apparatus specifically locates to the root of the primary dendrite, and its localization is already established in immature Purkinje cells that have multiple neurites. Inhibiting secretory trafficking through the Golgi apparatus reduces dendritic growth, suggesting that the Golgi apparatus is involved in the dendritic morphogenesis. We also demonstrated that in a mutant of an atypical protein kinase C (aPKC), Prkci, Purkinje cells retain multiple primary dendrites and show disrupted localization of the Golgi apparatus. Furthermore, a mosaic inhibition of Prkci in Purkinje cells recapitulates the aPKC mutant phenotype. These results suggest that the aPKC cell autonomously controls the Golgi localization and thereby regulates the specification of the primary dendrite of Purkinje cells.
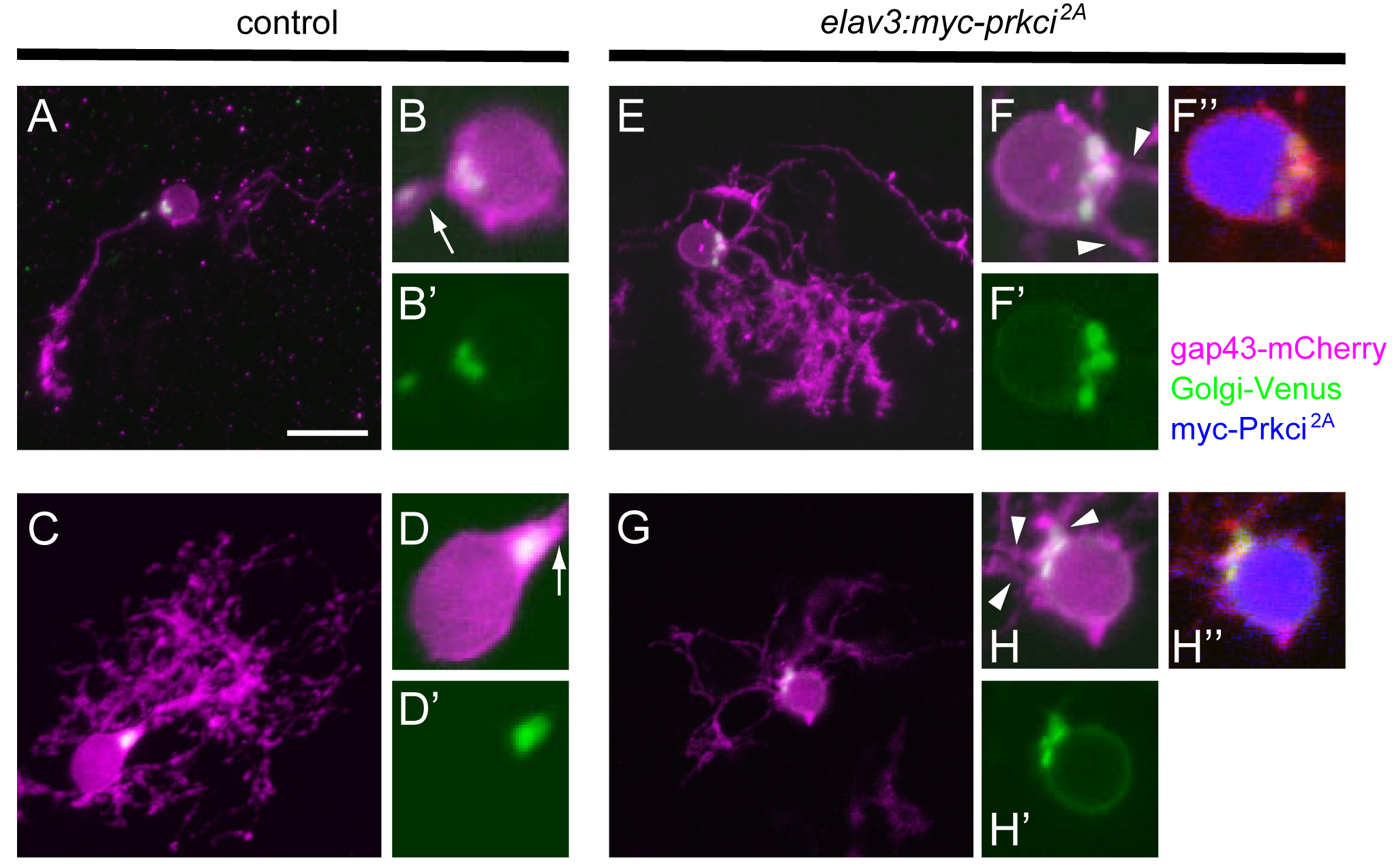
Prkci is required cell-autonomously for establishing the proper Golgi localization and selection of the primary dendrite. Purkinje cells from 5-dpf larvae expressing aldocl:gap43-mCherry-PTV1-2A-Golgi-Venus alone or with elavl3:myc-prkci2A. Control Purkinje cells extended a single primary dendrite, and Golgi-Venus (green) was exclusively localized to the root of a single primary dendrite (arrows). Purkinje cells that expressed dominant negative Prkci (myc-Prkci2A, blue) extended multiple primary dendrites, and the Golgi-Venus+ region was expanded. Extra primary dendrites were extended from adjacent sites to the expanded Golgi (arrowheads).
Zinc finger genes Fezf1 and Fezf2 control neuronal differentiation by repressing Hes5 expression in the forebrain [Pubmed]
Precise control of neuronal differentiation is necessary for generation of a variety of neurons in the forebrain. However, little is known about transcriptional cascades, which initiate forebrain neurogenesis. Here we show that zinc finger genes Fezf1 and Fezf2, which encode transcriptional repressors, are expressed in the early neural stem (progenitor) cells and control neurogenesis in mouse dorsal telencephalon. Fezf1- and Fezf2-deficient forebrains display upregulation of Hes5 and downregulation of neurogenin 2, which is known to be negatively regulated by Hes5. We show that FEZF1 and FEZF2 bind to and directly repress the promoter activity of Hes5. In Fezf1- and Fezf2-deficient telencephalon, the differentiation of neural stem cells into early-born cortical neurons and intermediate progenitors is impaired. Loss of Hes5 suppresses neurogenesis defects in Fezf1- and Fezf2-deficient telencephalon. Our findings reveal that Fezf1 and Fezf2 control differentiation of neural stem cells by repressing Hes5 and, in turn, by derepressing neurogenin 2 in the forebrain.
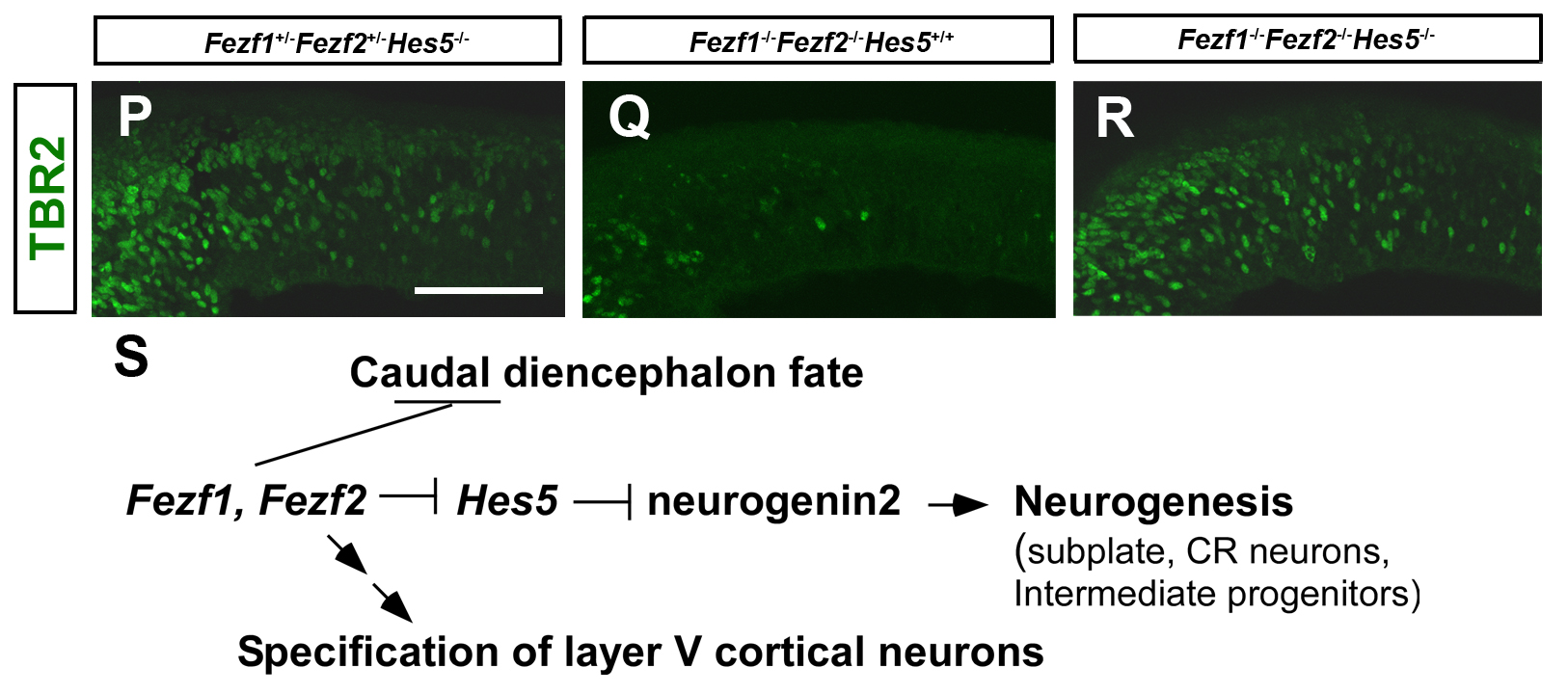
Immunostaining with anti-TBR2 antibody of E11.5 Fezf1+/- Fezf2+/- Hes5-/- (P), Fezf1-/- Fezf2-/- Hes5+/+, and Fezf1-/- Fezf2-/- Hes5-/- telencephalons. Coronal sections. TBR2+ intermediate progenitors were restored in Fezf1-/- Fezf2-/- Hes5-/- telencephalons. Schematic diagram of the roles of Fezf1 and Fezf2 in forebrain development.
Proneural gene-linked neurogenesis in zebrafish cerebellum [Pubmed]
In mammals, cerebellar neurons are categorized as glutamatergic or GABAergic, and are derived from progenitors that express the proneural genes atoh1 or ptf1a, respectively. In zebrafish, three atoh1 genes, atoh1a, atoh1b, and atoh1c, are expressed in overlapping but distinct expression domains in the upper rhombic lip (URL): ptf1a is expressed exclusively in the ventricular zone (VZ). Using transgenic lines expressing fluorescent proteins under the control of the regulatory elements of atoh1a and ptf1a, we traced the lineages of the cerebellar neurons. The atoh1+ progenitors gave rise not only to granule cells but also to neurons of the anteroventral rhombencephalon. The ptf1a+ progenitors generated Purkinje cells. The olig2+ eurydendroid cells, which are glutamatergic, were derived mostly from ptf1a+ progenitors in the VZ but some originated from the atoh1+ progenitors in the URL. In the adult cerebellum, atoh1a, atoh1b, and atoh1c are expressed in the molecular layer of the valvula cerebelli and of the medial corpus cerebelli, and ptf1a was detected in the VZ. The proneural gene expression patterns coincided with the sites of proliferating neuronal progenitors in the adult cerebellum. Our data indicate that proneural gene-linked neurogenesis is evolutionarily conserved in the cerebellum among vertebrates, and that the continuously generated neurons help remodel neural circuits in the adult zebrafish cerebellum.
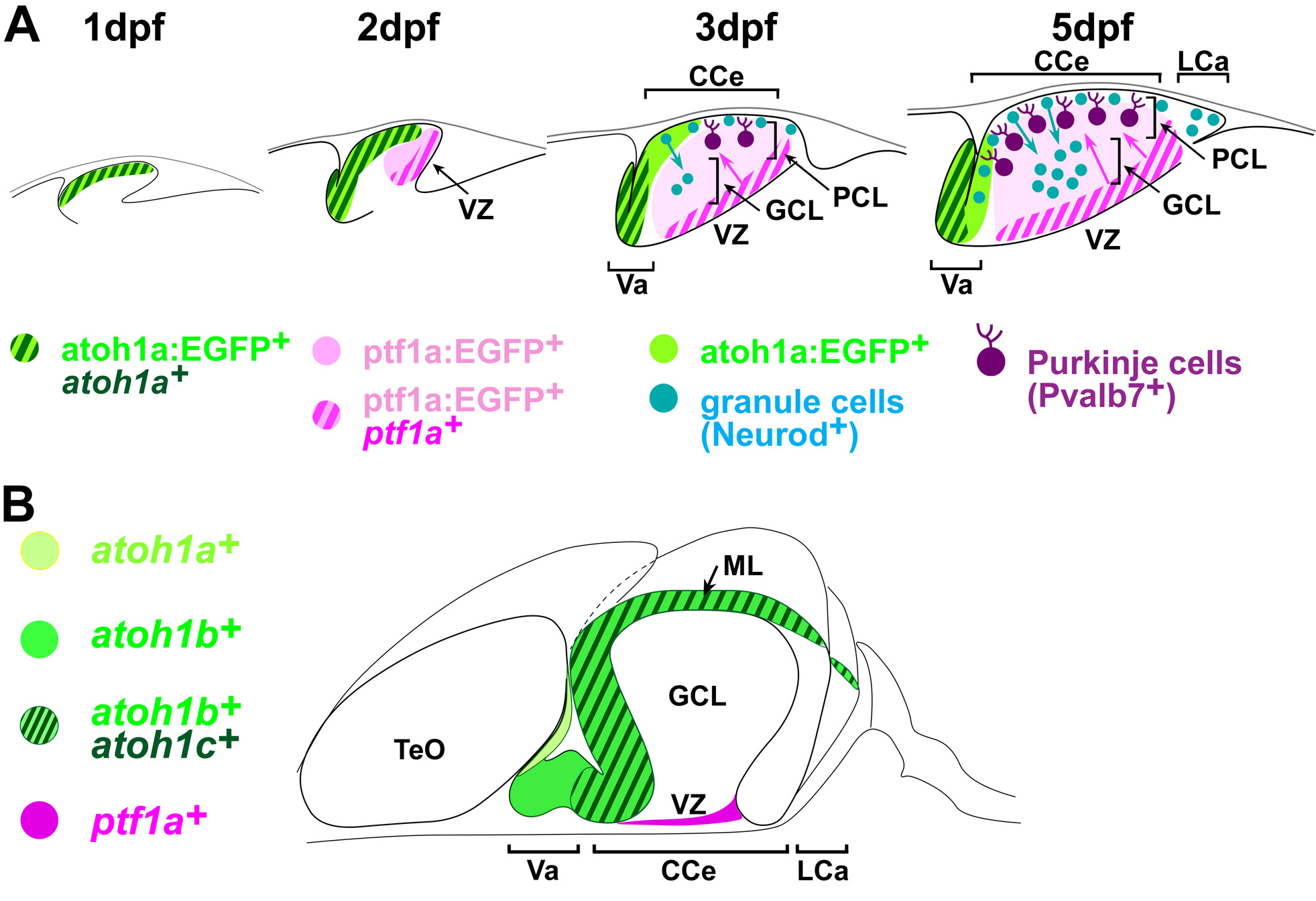
(A) Development of cerebellar neurons in zebrafish. At 1 day post fertilization (pdf), atoh1a-expressing cells form in the upper rhombic lip. At 2 dpf, atoh1a+ glutamatergic progenitors and ptf1a+ GABAergic progenitors occupy the rostral and caudal half of the cerebellum primordium. During 3-5 dpf, atoh1a+ progenitors give rise to Neurod1+ immature granule cells in the molecular layer (ML) and they migrate ventrally to form granule cell layer (GCL). ptf1a+ progenitors give rise to Purkinje cells at the ventricular zone (VZ) and they migrate dorsally to form Purkinje cell layer (PCL). (B) Expression of proneural genes in adult cerebellum. The proneural genes are expressed in proliferating neuronal progenitors. CCe: corpus cerebellaris, LCa: lobus caudalis cerebelli, TeO: optic tectum, Va: valvular cerebelli.
Anatomy of zebrafish cerebellum and screen for mutations affecting its development [Pubmed]
The cerebellum is important for the integration of sensory perception and motor control, but its structure has mostly been studied in mammals. Here, we describe the cell types and neural tracts of the adult zebrafish cerebellum using molecular markers and transgenic lines. Cerebellar neurons are categorized to two major groups: GABAergic and glutamatergic neurons. The Purkinje cells, which are GABAergic neurons, express parvalbumin7, carbonic anhydrase 8, and aldolase C like (zebrin II). The glutamatergic neurons are vglut1+ granule cells and vglut2high cells, which receive Purkinje cell inputs; some vglut2high cells are eurydendroid cells, which are equivalent to the mammalian deep cerebellar nuclei. We found olig2+ neurons in the adult cerebellum and ascertained that at least some of them are eurydendroid cells. We identified markers for climbing and mossy afferent fibers, efferent fibers, and parallel fibers from granule cells. Furthermore, we found that the cerebellum-like structures in the optic tectum and antero-dorsal hindbrain show similar Parvalbumin7 and Vglut1 expression profiles as the cerebellum. The differentiation of GABAergic and glutamatergic neurons begins 3 days post-fertilization (dpf), and layers are first detectable 5 dpf. Using anti-Parvalbumin7 and Vglut1 antibodies to label Purkinje cells and granule cell axons, respectively, we screened for mutations affecting cerebellar neuronal development and the formation of neural tracts. Our data provide a platform for future studies of zebrafish cerebellar development.
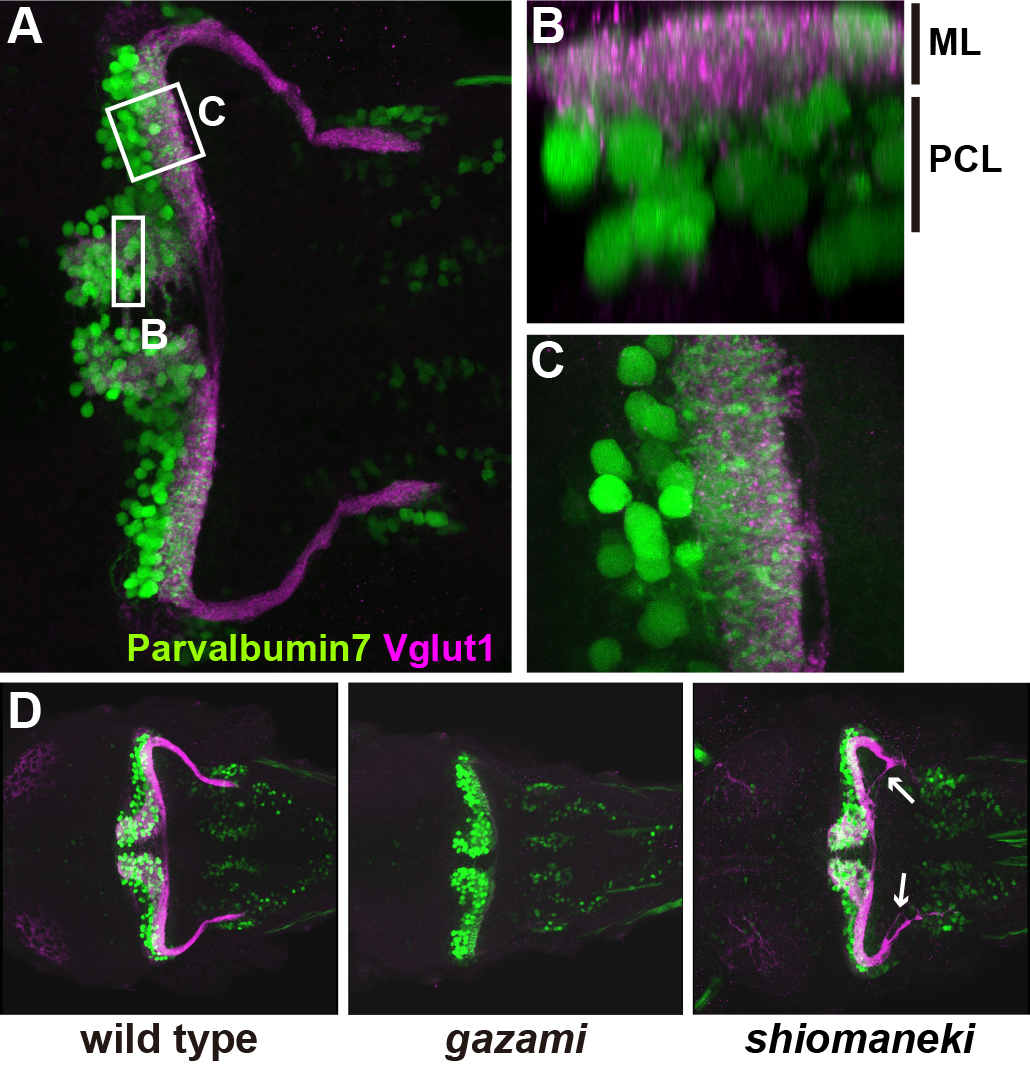
Early stage larvae (5 days post fertilization: dpf). (A-C) A larva is stained with anti-Parvalbumin7 (Purkinje cells) and anti-Vglut1 (granule cell axons) antibodies. Optical section of box B and higher magnification view of C. At 5 dpf, the molecular layer (ML) and Purkinje cell layer (PCL) are observed. (D) Wild-type, gazami, and shiomankei mutant larvae. gazami mutant larvae show defects in granule cell axons. shiomaneki mutant larvae abnormality in axogenesis of granule cells.