Topics
Axis formation
Research achievements
(1) Mechanisms of dorsal organizer formation
In amphibian and teleost embryos, the dorsal determinants are believed to be transported from their initial location in the vegetal pole to the prospective dorsal side of the embryos. The dorsal determinants are known to activate the canonical Wnt pathway and thereby promote the expression of genes involved in the induction of the dorsal organizer. The zebrafish homeobox gene dharma (dha, also known as bozozok) and a nodal-related gene ndr1 (also known as squint) function downstream of the maternally derived Wnt signal, and are required for formation of the dorsal organizer.
We found that Dharma functions as a transcriptional repressor and represses the expression of ventrally-expressed homeobox genes vox, vent and ved. Vox, Vent, and Ved also act as transcriptional repressors that inhibit expression of organizer genes. Our data indicate that Dharma-mediated repression of vox, vent and ved restricts the dorsal organizer domain
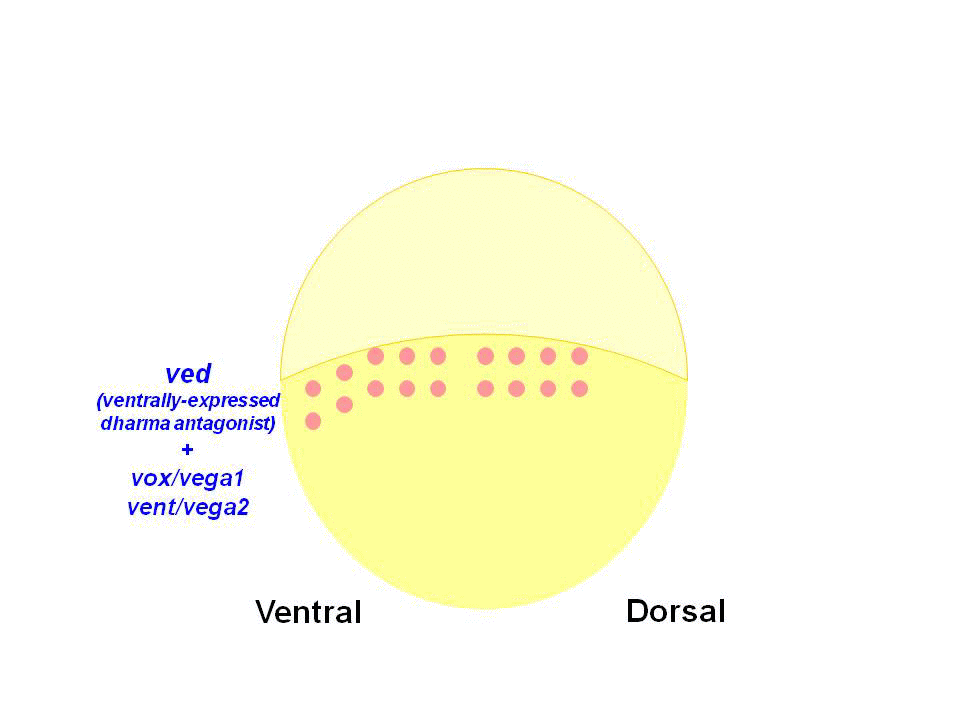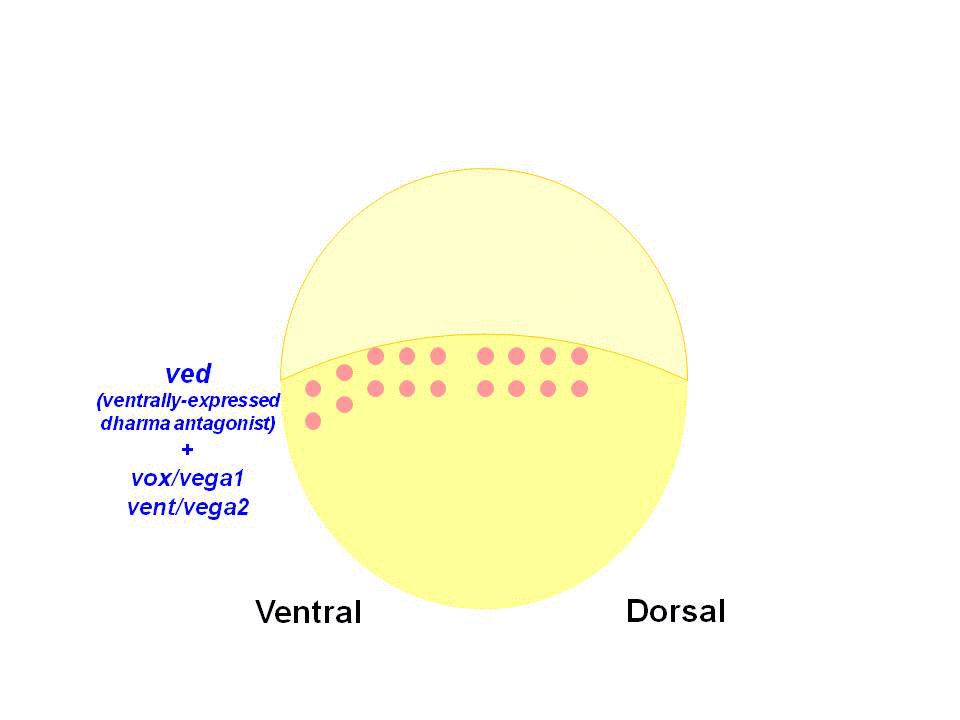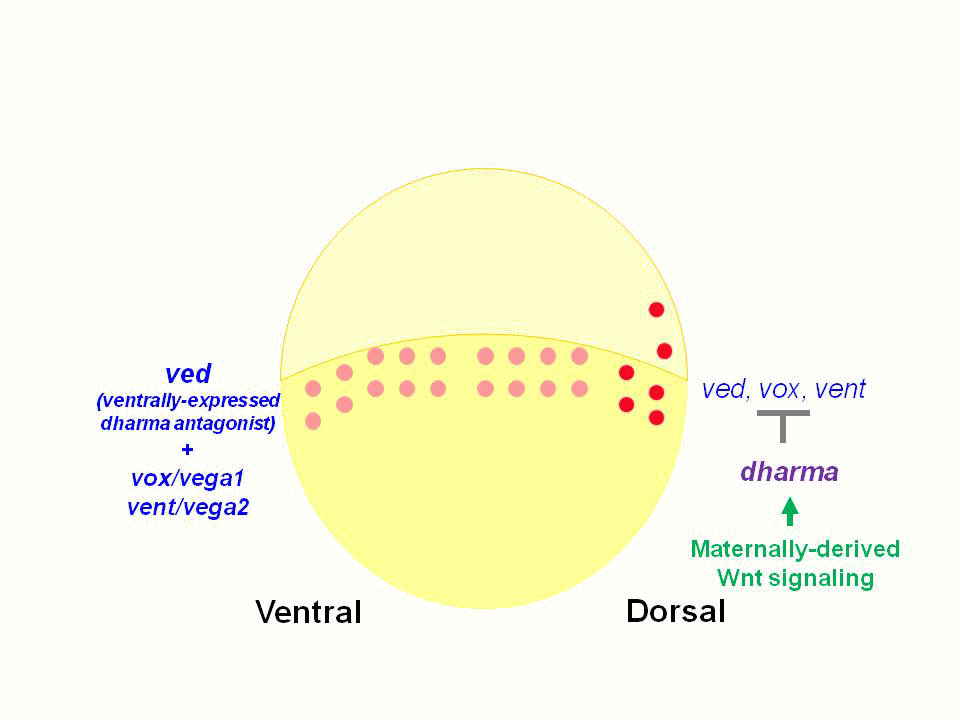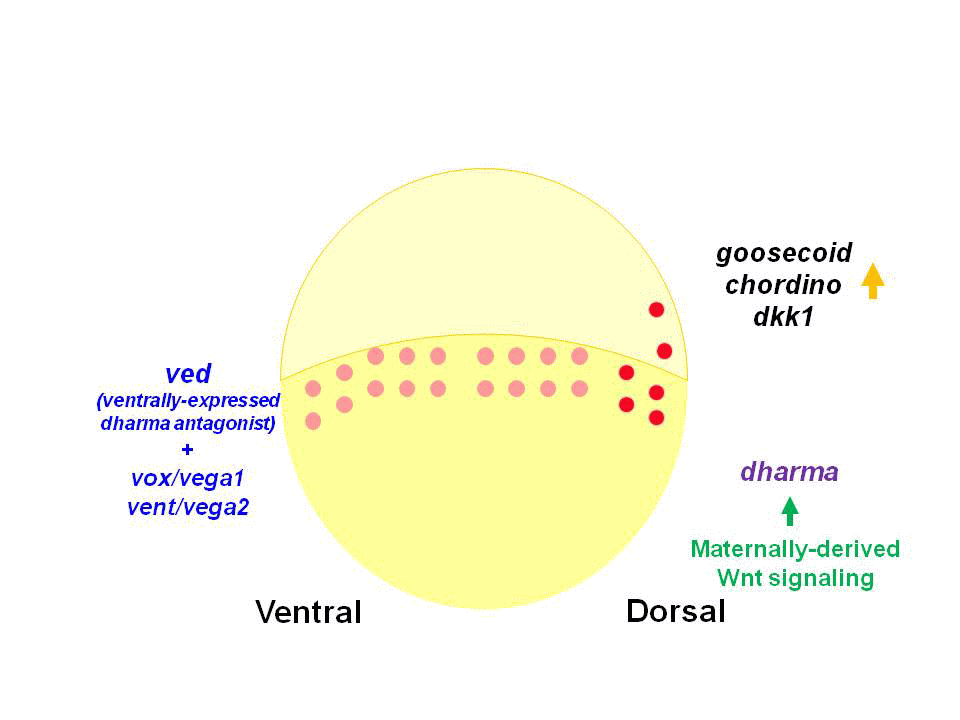
(2) Regulation of dorso-ventral polarity by Secreted Frizzled (Sizzled)
The zebrafish mutant ogon displays ventralized phenotypes similar to the chordin mutant, in which the gene for the Bmp antagonist Chordin is mutated. We isolated the gene responsible for ogon by a positional cloning strategy and found that the ogon locus encodes a zebrafish homologue of Secreted Frizzled (Sizzled), which has sequence similarity to a Wnt receptor, Frizzled. sizzled expression on the ventral side was dependent on Bmp signaling. Our data indicated that Sizzled functions as a negative feedback regulator of Bmp signaling and that Sizzled is required to suppress the Bmp signal on the ventral side of zebrafish embryos. We found that Sizzled stabilizes Chordin by binding to and inhibiting the Tolloid-family metalloproteinase Bmp1, which cleaves and inactivates Chordin. Our results revealed that Sizzled represses the activities of Tolloid-family proteins, thereby creating a Chordin-Bmp activity gradient along the dorso-ventral axis.
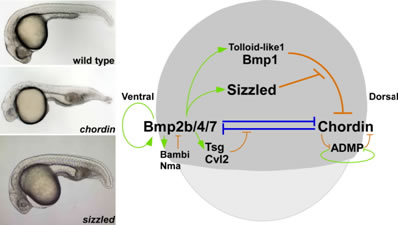
Sizzled protects Chordin, a Bmp antagonist, by inhibiting the Tolloid-family metalloproteinase Bmp1/Tolloid-like1, which cleave and inactivate Chordin. Phenotypes of wild-type and, chordin and sizzled mutants
(3) Left-right axis formation
Left-right axis formation relies on the nodal flow, in which the cilia generates a leftward flow by their rotation in the mouse node or the fish Kupffer’s vesicle during the early segmentation period. We identified charon, which encodes a member of the Cerberus/Dna family of secreted factors. charon is expressed in the cells embracing Kupffer’s vesicle. We found that Charon inhibits Nodal signaling and thereby suppress the left side formation on the right side. We also studied a medaka mutant mii, which exhibits defects in the left-right polarity of organs, and found that mii encodes dynein axonemal intermediate chain 2a (dnai2a). Our findings demonstrate that Dnai2a controls left-right polarity through regulation of ciliary motility.
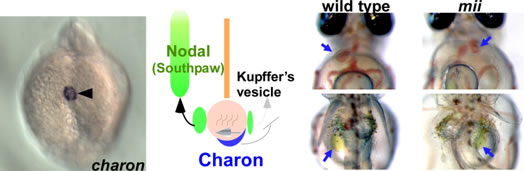
Expression of zebrafish charon (left panel) and phenotype of medaka mii mutant (right panel). charon is expressed in the cells embracing Kupffer’s vesicle and Charon function s to suppress left-side formation on the right side. Some of the medaka dnai2a mutant mii show situs inversus.
Current project
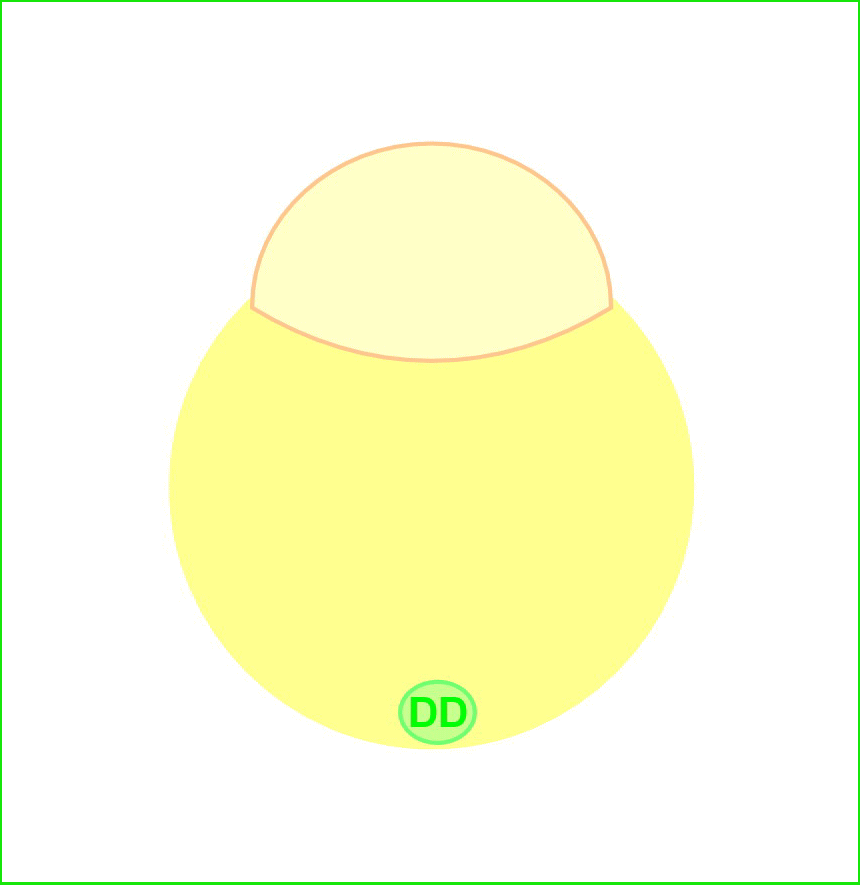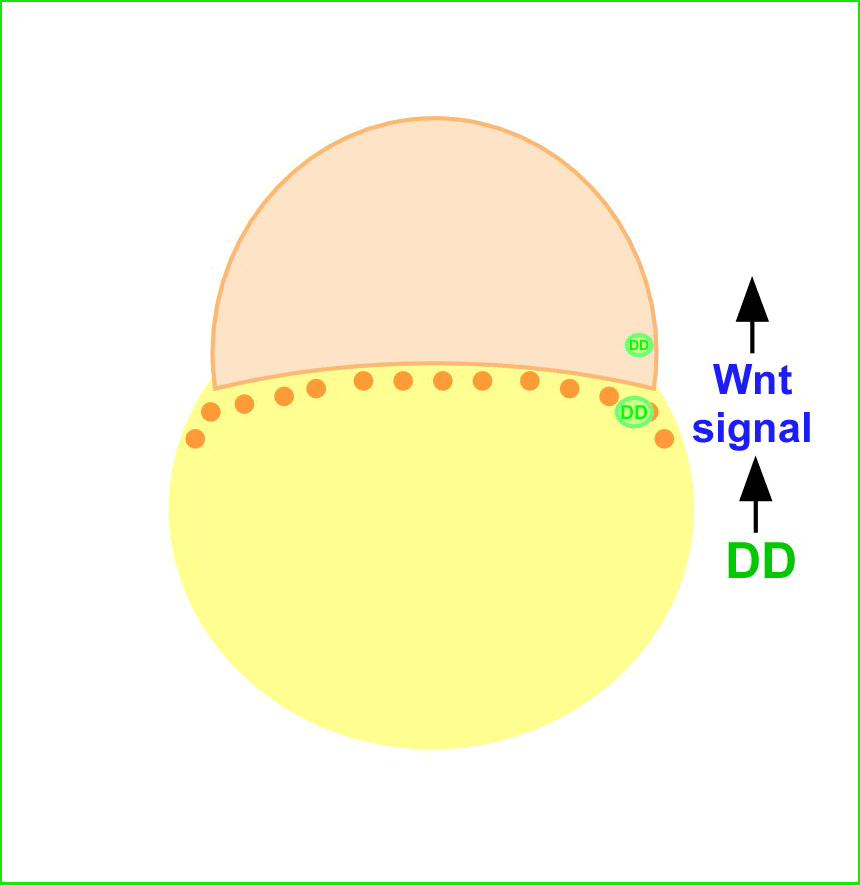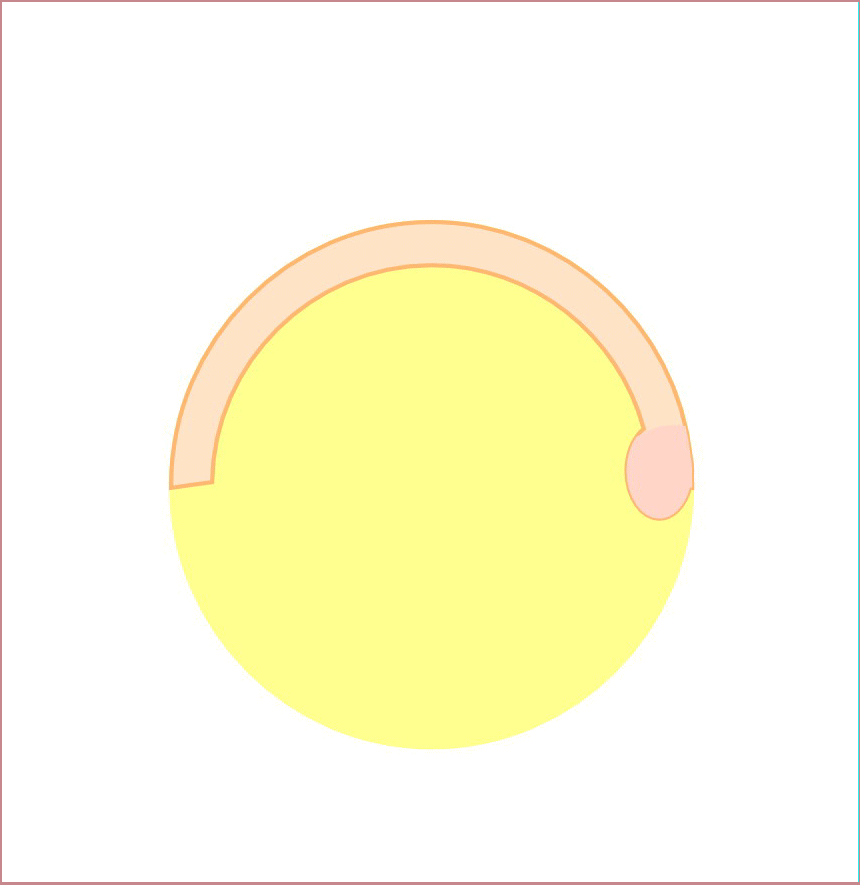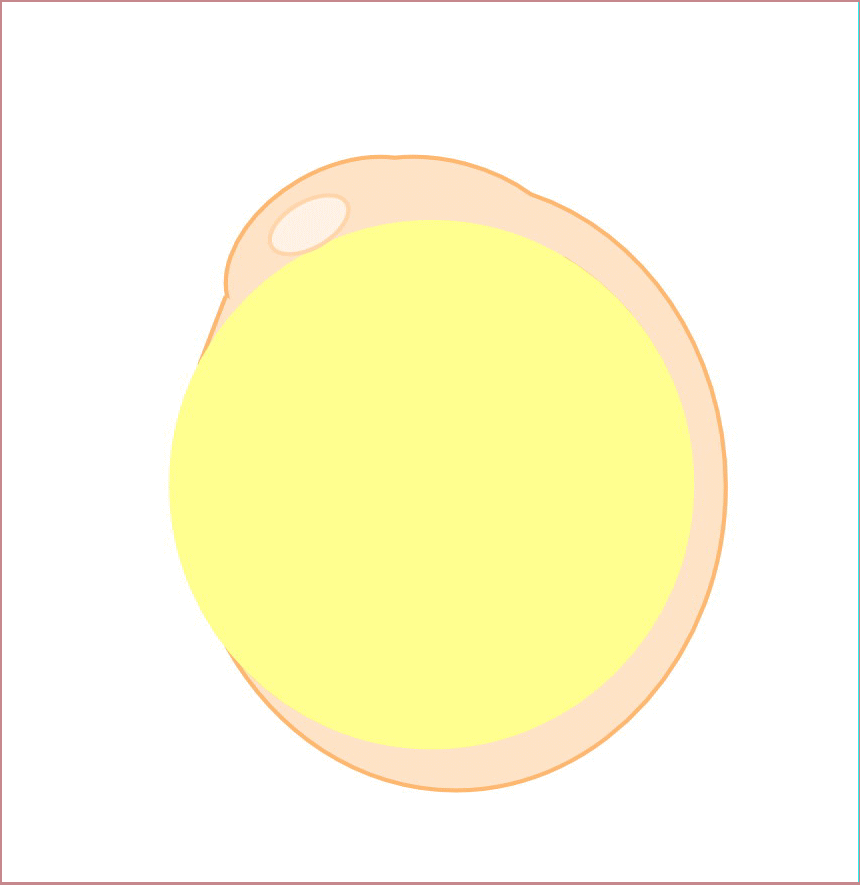
We previously identified a novel maternal-effect recessive mutation, tokkaebi that affects the formation of dorsal structures, leading to ventralization of the embryos. Severely ventralized phenotypes, including the loss of dorso-anterior structures, were seen in 5-100% of the embryos obtained from tokkaebi homozygous females. The tokkaebi embryos displayed defects in the nuclear accumulation of β-catenin on the dorsal side, and the reduced or non-expression of dorsal-specific genes. These data suggested that maternally derived Wnt signaling is specifically affected in the tokkaebi mutants. By identifying the tokkaebi locus, we found that Syntabulin, a linker of the kinesin I motor protein, is essential for dorsal determination in zebrafish. We demonstrate that Syntabulin is translocated from the vegetal pole in a microtubule-depedent manner. Our findings suggest that Syntabulin regulates the microtubule-dependent transport of the dorsal determinants. We are trying to discover the dorsal determinants and to reveal the mechanism that controls the formation of the uni-directional microtubule array.
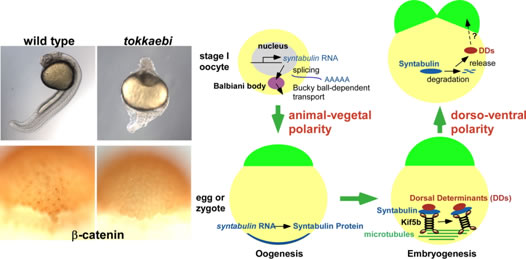
Syntabulin is involved in the dorsal determination. Embryos obtained from tokkaebi homozygous females show severely ventralized phenotypes. Accumulation of β-catenin in the dorsal nuclei at mid-blastula stages is impaired in tokkaebi embryos (left panels). Schematic presentation for Syntabulin’s role in dorsal determination (right panel). During oogenesis, syntabulin RNA is transported from the nucleus to the vegetal pole through the Buc-mediated Balbiani body-dependent pathway. During early embryogenesis, Syntabulin links the dorsal determinants to Kif5B, the heavy chain of kinesin I. At 20 minute post fertilization (mpf), a microtubule array forms and Syntabulin transports the dorsal determinants to the plus end of microtubules. Around the 2-cell stage, Syntabulin is degraded and releases the DDs, which are eventually incorporated by dorsal blastomeres and activate zygotic gene expression cascades required for the formation of the dorsal organizer.







