Topics
Neural development
Research achievements
(1) Roles of caudal-related genes cdx1a and cdx4 in the formation of posterior neural tissue
We studied the role of Wnt signaling in the posterior body formation. In zebrafish, wnt3a and wnt8 are expressed in overlapping domains in the blastoderm margin and later in the tailbud. The combined inhibition of Wnt3a and Wnt8 leads to loss of the posterior structure. We found that expression of the caudal-related cdx1a and cdx4 is regulated by Wnt signaling. Like the wnt3a/wnt8-defective embryos, cdx1a/cdx4-defective embryos display complete loss of the tail structure, indicating that the cdx genes mediate Wnt signaling and play an essential role in the morphogenesis of the posterior body in zebrafish. We further found that the inhibition of cdx1a and cdx4 caused the ectopic expression of genes that are normally expressed in the posterior hindbrain and anterior spinal cord and the ectopic formation of hindbrain motor and commissure neurons in the posteriormost neural tissue. Fibroblast growth factor (Fgf) and retinoic acid (RA) signals are required for the ectopic expression. Our data suggest that Cdx suppresses the posterior hindbrain fate by modifying the tissue competence to respond to Fgfs and RA in neural tissue.
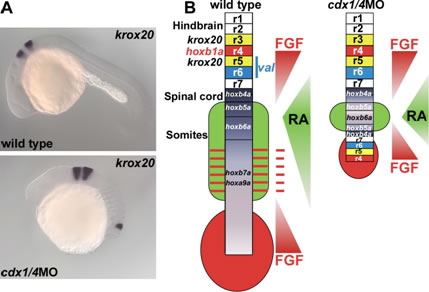
Ectopic formation of hindbrain in cdx1a/4-deficient embryos. (A) Ectopic expression of krox20 is detected in the posteriormost neural tissue of a cdx1a/4-deficient embryo. (B) Schematic representation of Fgf and RA signaling gradients and neural structures in wild-type and cdx1a/4-deficient embryos.
(2) Mechanisms controlling primary neurogenesis
In teleosts and amphibians, the proneuronal domains, which give rise to primary-motor, primary-inter and Rohon-Beard (RB) neurons, are established at the beginning of neurogenesis as three longitudinal stripes along the anteroposterior axis in the dorsal ectoderm. The proneuronal domains are prefigured by basic helix-loop-helix (bHLH) proneural genes, and separated by domains (inter0proneuronal domains) that do not express the proneuronal genes. We found that the zebrafish hairy- and enhancer of split-related (Her) genes her3 and her9 are expressed in the inter-proneuronal domains, and are required for their formation. her3 and her9 expression is not regulated by Notch signaling, but rather is controlled by positional cues, in which Bmp signaling is involved. The combined inhibition of Her3 and Her9 induced ubiquitous expression of proneural and neuronal genes in the neural plate, and abolished the formation of the inter-proneuronal domains. Our data indicate that her3 and her9 function as pre-patterning genes that link the positional dorso-ventral polarity information in the posterior neuroectoderm to the spatial regulation of neurogenesis.
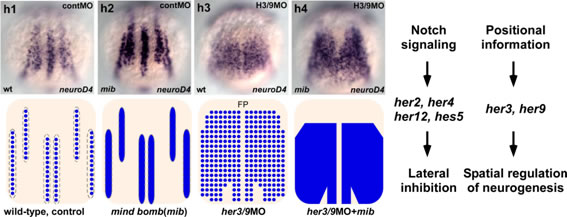
her3 and her9 function as prepattern genes. Expression of the proneural gene neurod4 in a control embryo, Notch signal-defective embryo (mib: mind bomb mutant), her3/her9-defective embryo, and Notch signal and her3/her9-defective embryo. (Right) Roles of her3/her9, and of her genes whose products function downstream of Notch signaling in neurogenesis.
(3)Formation of the forebrain and olfactory system by zinc-finger genes Fezf1 and Fezf2
We previously isolated a zebrafish zinc finger gene, fezf2 (fezl), which is expressed in the forebrain. It has sequence similarity to Xenopus Fezf1 (Fez) and encodes a transcriptional repressor. In collaboration with Dr. Rosenthal’s laboratory, we found that a mutation in the fezl gene was responsible for the zebrafish mutant too few, which displays a reduction or loss of monoaminergic (dopaminergic and serotonergic) neurons in the basal forebrain. We isolated mouse Fezf1 and Fezf2 cDNAs and found that they are expressed in subdomains of the forebrain and in the olfactory epithelium. We then generated Fezf1 and Fezf2-deficient mice, and Fezf1;Fezf2-double deficient mice and studied the roles of these molecules in the formation and function of the forebrain and olfactory sensory system.
We found that Fezf2 is involved in the differentiation of subplate neurons and the formation of the fimbria and fornix, and Fezf2 is essential for the specification of the subcerebral projection neurons in the neocortex. We also found that Fezf1 is cell-autonomously required for the axon termination of the olfactory sensory neurons, and Fezf1 non-cell-autonomously regulates layer formation and interneuron development in the olfactory bulb. The Fezf1;Fezf2-deficient mice show abnormalities in the subdivision of the diencephalon. We revealed that Fezf1 and Fezf2 repress the posterior diencephalic fate in the anterior diencephalon and the telenecephalon. The Fezf1;Fezf2-deficient mouse forebrains display upregulation of Hes5 and downregulation of neurogenin 2, which is known to be negatively regulated by Hes5. In the Fezf1;Fezf2-deficient mouse forebrains, the differentiation of neural stem cells into early-born cortical neurons and intermediate progenitors is impaired. Our finding reveal that Fezf1 and Fezf2 control differentiation of neural stem cells by repressing Hes5 and, in turn, by depressing neurogenin2 in the forebrain.
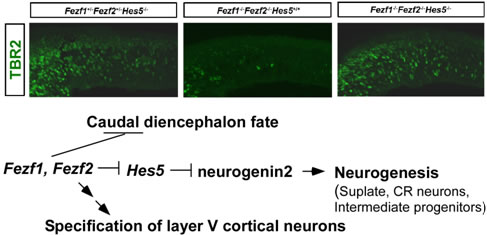
Roles of Fezf1 and Fezf2 in forebrain development. TBR2+ intermediate progenitors are reduced in Fezf1-/-Fezf2-/- telencephalons, but are restored in Fezf1-/-Fezf2-/-Hes5-/- telencephalons.
Current projects
We are investigating the formation and function of cerebellar neural circuits using zebrafish and medaka. The cerebellum, a structure derived from the dorsal part of the most anterior hindbrain, functions in the control of smooth and skillful movements. The cerebellum is also involved in higher cognitive and emotional functions. While the structure and development of the cerebellum have been analyzed most extensively in mammals, recent studies have shown that the anatomy and development of the cerebellum is conserved between mammals and teleost species, including zebrafish and medaka. Purkinje and granule cells serve, respectively, as the major GABAergic and glutamatergic neurons. Purkinje cells are derived from ptf1a-expressing neuronal progenitors in the ventricular zone (VZ), receive inputs from climbing fibers from the inferior olive nuclei. Granule cells are derived from atoh1a/b/c-expressing progenitors in the upper rhombic lip (URL) receive inputs from mossy fibers. Information from mossy fibers is conveyed to the dendrites of Purkinje cells by the axons of granule cells, called parallel fiber. The information of from climbing and mossy fibers is integrated by Purkinje cells. The precise control of the cerebellar neural circuitry formation is required to elicit motor control and modulate higher cognitive/emotional functions. We are investing the development and functions of the cerebellar neural circuits.
During the development of Purkinje cells, we found that immature Purkinje cells initially extend multiple neurites from the soma and subsequently retract all but one, which becomes the primary dendrites. The Goligi apparatus specifically locates to the root of the primary dendrite, and its localization is already established in immature Purkinje cells that have multiple neurites. We recently demonstrated that the atypical protein kinase C (aPKC) Prkci cell autonomously controls the Golgi localization and thereby regulates the specification of the primary dendrite of Purkinje cells.
The differentiation of GABAergic and glutamatergic neurons began at 3 dpf, and the layers (molecular layer, Purkinje cell layer and granule cell layer) were first detected at 5 dpf in zebrafish. We screened for zebrafish mutations affecting cerebellar neuronal development and the formation of neural tracts. We are also studying a medaka mutant, rolling, which shows ataxic phenotypes. By identifying mutant loci, we will reveal genetic programs that control the development and functions of cerebellar neural circuits. We will also analyze the functions of the cerebellar neural circuits using transgenic zebrafish and medaka
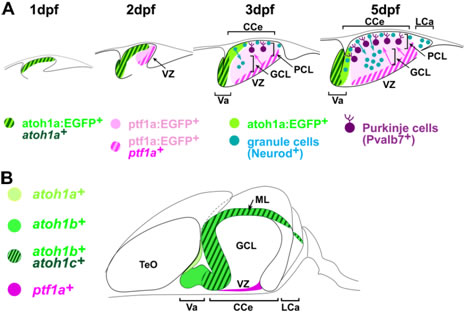
Development of cerebellar neurons. We studies development of the glutamatergic and GABAergic lineages using the zebrafish transgenic lines, Tg(atoh1a:EGFP) and Tg(ptf1a:EGFP). (A) At 2 dpf, atoh1a+ glutamatergic and ptf1a+ GABAergic progenitors occupy, respectively, the anterior and posterior domains, of the cerebellum. From 3 to 5 dpf, atoh1a+ progenitors generate atoh1a:EGFP+ granule cells in the molecular layer (ML), which become Neurod+ immature granule cells and migrate to the granule cell layer (GCL); ptf1a:EGFP+ cells migrate dorsally from the VZ and become differentiated into Purkinje cells at the Purkinje cell layer. These processes generate the three cerebellar layers visible at 5 dpf. (B) Expression of atoh1a, atoh1b, atoh1c, and ptf1a in the zebrafish adult cerebellum. They are expressed in proliferating neuronal progenitors. CCe, corpus cerebelli; LCa, Lobus caudalis cerebelli; TeO, Tectum opticum; Va, valvula cerebelli.
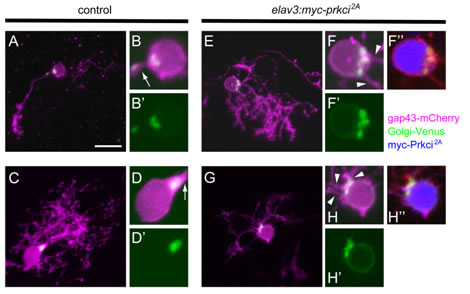
Prkci is required cell autonomously for establishing the proper Golgi localization and selection of the primary dendrite. Purkinje cells from 5 dpf larvae expressing aldoca:gap43-mCherry-PTV1–2A-Golgi-Venus alone (A–D, control) or with elavl3:myc-prkci2A (E–H). All are dorsal view projected images. Control Purkinje cells extended a single primary dendrite, and Golgi-Venus (green) was exclusively localized to the root of a single primary dendrite (arrows). Purkinje cells that expressed myc-Prkci 2A (blue) extended multiple primary dendrites, and the Golgi-Venus+ region was expanded. Extra primary dendrites were extended from adjacent sites to the expanded Golgi (arrowheads).







